You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Enthalpy of Subcooled Liquid
- Thread starter Viper5
- Start date

Help Support Professional Engineer & PE Exam Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
JHW 3d
Here's Johnny...
For subcooled water, is the enthalpy value obtained by looking up saturation pressure or temperature? Thanks.
Once you move outside the vapor dome, pressure and temperature are not constant. Your state enthalpy will consist of saturated liquid enthalpy at pressure equal to your state pressure and a cp*DeltaT term at the sub cooled temperature difference from the sat liquid temp.
(I think this is correct)
JHW 3d
Here's Johnny...
1. Find enthalpy of sat liquid at 1atm.I'm not sure I understand. Suppose I wanted to find the enthalpy of water at P=1atm, T=60 deg C. How would one go about this?
2. Record T for state 1.
3. Calculate cp*(60C - T).
4. h_tot = Add 1 and 3.
I see where you are going with this. I think you meant sat liquid in part one since you are dealing with subcooled. What had me questioning this is one of the solutions in the Practice Problem book by Lindeburg just uses a T = T_sat assumption to find h directly and I wasn't sure why P= P_sat could be disregarded. It briefly states that h and v (nu) are independent of pressure. Thus, it uses the respective saturation temperature value. Maybe the solution is wrong.
JHW 3d
Here's Johnny...
Or I am wrong.I see where you are going with this. I think you meant sat liquid in part one since you are dealing with subcooled. What had me questioning this is one of the solutions in the Practice Problem book by Lindeburg just uses a T = T_sat assumption to find h directly and I wasn't sure why P= P_sat could be disregarded. It briefly states that h and v (nu) are independent of pressure. Thus, it uses the respective saturation temperature value. Maybe the solution is wrong.
Good catch yes I meant saturated liquid. I'll edit my answer.
Specify which answer in Lindbergh you are referring to and I will review it.
Per the MERM "A subcooled liquid is at a temperature less than the saturation temperature corresponding to its pressure. Unless the pressure of the liquid is very high, the various thermodynamic properties can be considered to be functions of only the liquid's temperature."
justin-hawaii
Justin PE
Viper5,
I think the best way to understand this topic is to look at the Pressure-Enthalpy diagram below. Pick a point in the subcooled region at any temperature and pressure (try 20 F and 60 PSIA). Next, try and find the enthalpy (it is around 20 Btu/lb). You will notice that in order to find the enthalpy of this point, you take a vertical line down to the enthalpy value. This enthalpy value is the same enthalpy value as a saturated liquid at the same temperature as your selected point in the subcooled region. Hopefully this helps to make more sense of things. I like to draw a rudimentary P-H diagram when doing these types of problems, if a P-H diagram is not available.
Justin
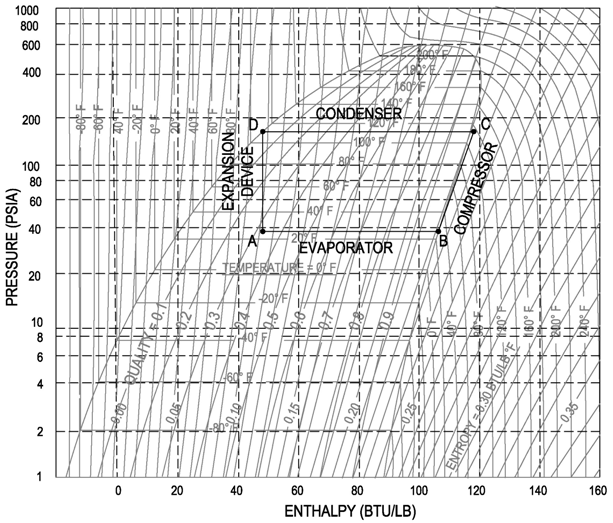
I think the best way to understand this topic is to look at the Pressure-Enthalpy diagram below. Pick a point in the subcooled region at any temperature and pressure (try 20 F and 60 PSIA). Next, try and find the enthalpy (it is around 20 Btu/lb). You will notice that in order to find the enthalpy of this point, you take a vertical line down to the enthalpy value. This enthalpy value is the same enthalpy value as a saturated liquid at the same temperature as your selected point in the subcooled region. Hopefully this helps to make more sense of things. I like to draw a rudimentary P-H diagram when doing these types of problems, if a P-H diagram is not available.
Justin

Similar threads
- Replies
- 2
- Views
- 1K
- Replies
- 1
- Views
- 409
- Replies
- 3
- Views
- 2K
Latest posts
-
-
-
-
For Sale Selling PE CIVIL Breadth and Construction Depth - EET Binders - 2023 Edition
- Latest: CivilEngineerXpert
-
-
-


